Cognitive dysfunctions in patients with obsessive–compulsive disorder compared to the patients with schizophrenia patients: Relation to overvalued ideas
Cognitive dysfunctions in patients with obsessive–compulsive disorder compared to the patients with schizophrenia patients:
Relation to overvalued ideas
Arzu Kitis a, Berna Binnur Kivircik Akdede a, Koksal Alptekin a,*,Yildiz Akvardar a, Almila Erol b, Nezaket Kaya c
Department of Psychiatry, Medical School of Dokuz Eylul University, Izmir, Turkey
Department of Psychiatry, Izmir Ataturk Education and Research Hospital, Izmir, Turkey
Department of Psychiatry, Izmir Education and Research Hospital, Turkey
Available online 17 August 2006
Abstract
Objective: The clinical overlaps between schizophrenia and obsessive–compulsive disorder (OCD) seem to be related to thought disorders involving obsessions, overvalued ideas, and delusions. Overvalued ideas are beliefs falling in between obsessions and delusions and are stronger than obsessions but weaker than delusions. The goal of the present study was to compare patients with OCD to those with schizophrenia in terms of cognitive functions and to relate cognition and overvalued ideas in OCD.
Methods: Twenty-three patients with OCD (free of depression), 24 patients with schizophrenia, and 22 healthy subjects matched to patients in age, gender, education, and hand dominance were included in the study. All subjects were administered neurocognitive tests assessing verbal learning- memory, executive functions, verbal fluency, attention and verbal working memory.
Results: Patients with schizophrenia showed worse performance on cognitive tests than the OCD and control groups. The severity of overvalued ideas was significantly correlated to cognitive functions in the OCD group. There were no significant differences in cognitive functions between schizophrenia group and the OCD patients who had higher scores on the Overvalued Ideas Scale (OVIS).
Conclusion: Overvalued ideas in OCD may be related to cognitive dysfunctions in OCD and this subtype of OCD may have similar characteristics to schizophrenia in terms of cognition.
© 2006 Elsevier Inc. All rights reserved.
Keywords: Cognition; Obsessive–compulsive disorder; Overvalued ideas; Schizophrenia
Abbreviations: ACTT, Auditory Consonant Trigram Test; CGI, Clinical Global Impression Scale; CWAT, Controlled Word Association Test; DSM-IV, Diagnostic and Statistical Manual of Mental Disorders-fourth edition; DST, Digit Span Test; HDRS, Hamilton Depression Rating Scale; HQ, Handedness Questionnaire; OCCL, Obsession Compulsion Check List; OCD, Obsessive– Compulsive Disorder; OCS, Obsessive–Compulsive Symptoms; OVIS, Over- valued Ideas Scale; PANSS, Positive and Negative Syndrome Scale; RAVLT, Rey Auditory Verbal Learning Test; TMT A–B, Trail-Making Test A–B; WCST, Wisconsin Cart Sorting Test; Y-BOCS, Yale–Brown Obsessive Compulsive Scale.
* Corresponding author: Dokuz Eylül Üniversitesi Tıp Fakültesi, Psikiyatri Bölümü 35340 Balçova, İzmir, Turkey. Tel.: +90 232 4124157 fax: +90 232 2788191 or +90 232 4124169.
* E-mail adress:[email protected] (K. Alptekin).
0278-5846/$ - see front matter © 2006 Elsevier Inc. All rights reserved. doi: 10.1016/j.pnpbp.2006.06.022
1.Introduction
Studies comparing patients with schizophrenia and those with obsessive–compulsive disorder (OCD) have shown similarities and overlaps as well as differences. The overlaps between two disorders seem to be related to thought disorders involving obsessions, overvalued ideas and delusions. As some OCD patients suffer from obsessions and compulsions that resemble psychosis, a special subcategory for OCD has been delineated as “OCD with poor insight” in DSM-IV (Foa, 1979; Lelliott et al., 1988; Neziroglu and Yaryura-Tobias, 1995). Although DSM-IV classification regards OCD with poor insight essentially as an anxiety disorder, some authors have called attention to the delusional qualities of “OCD with poor insight”, emphasized its psychotic dimension, and labeled it as a “Schizo-obsessive subtype”, “Schizo-obsessive Disorder” or an “Obsessive Psycho- sis” (Hwang and Hollander, 1993; Payurovsky et al., 2003).
Sometimes obsessions may evolve, intensify, and change to overvalued ideas. These ideas are then viewed by the patients as realistic, are associated with poor insight, and are prognostically related to worse outcomes. OCD patients with overvalued ideas no longer view these obsessions as senseless, unwanted, or uncontrollable because their “insight” has deteriorated. Overvalued ideas in OCD are conceptualized as falling between the extreme points of a continuum that ranges from the rational thought process with obsessions to a blatantly delusional ideation (Kozak and Foa, 1994; Neziroglu et al., 1999).
Co-existence of obsessions and compulsions in schizophrenia has been discussed in psychiatric publications since the turn of the 20th century. Studies of obsessions and compulsions in schizophrenia found that the frequency ranges from 3.5% to 60% (Rosen, 1957; Fenton and McGlashan, 1986; Bland et al., 1987; Eisen and Beer, 1997; Payurovsky and Fuchs, 1999). Reasons for the extremely wide range of these estimates may lie in the differences both in sampling strategies and in methodology of determining the presence/absence of these symptoms. Eisen and Rasmussen (1993) detected psychotic symptoms in 14% of the 475 patients with OCD and showed that 4% (18 of 475) met full diagnostic criteria for schizophrenia. An epidemiological review study by Tibbo and Warneke (1999) reports the co-morbidity rate of 12.2% for OCD and schizophrenia.
In schizophrenia, cognitive impairment has been reported for several aspects of cognition such as attention, verbal memory and executive functions (Saykin and Shtasel, 1994; Abbruzzese and Bellodi, 1995; Abbruzzese et al., 1997; Gold and Carpenter, 1997; Bilder et al., 2000). Andreasen and her team (1996, 1999) have suggested that patients with schizophrenia suffer from cognitive dysmetria and hypothesized that schizophrenia presents as a cognitive disorder involving the cortical–thalamic–cerebellar–cortical circuit.
The various cognitive tests used, the cognitive function each of these tests measures, and the results of the various studies on OCD patients are summarized in Table 1.
When the disease severity and the neurocognitive functions in patients with schizophrenia without OCD are compared to those with schizophrenia and OCD, the course of the disease is more severe in those with OCD than in those without OCD (Hwang et al., 2000) and the abnormalities in cognitive functions are more evident (Hwang et al., 2000; Lysaker et al., 2000, 2002).
The aim of the present study was to compare cognitive functioning of patients with obsessive compulsive disorder to its counterpart in patients with schizophrenia and to examine the relationship between cognitive functioning and overvalued ideas.
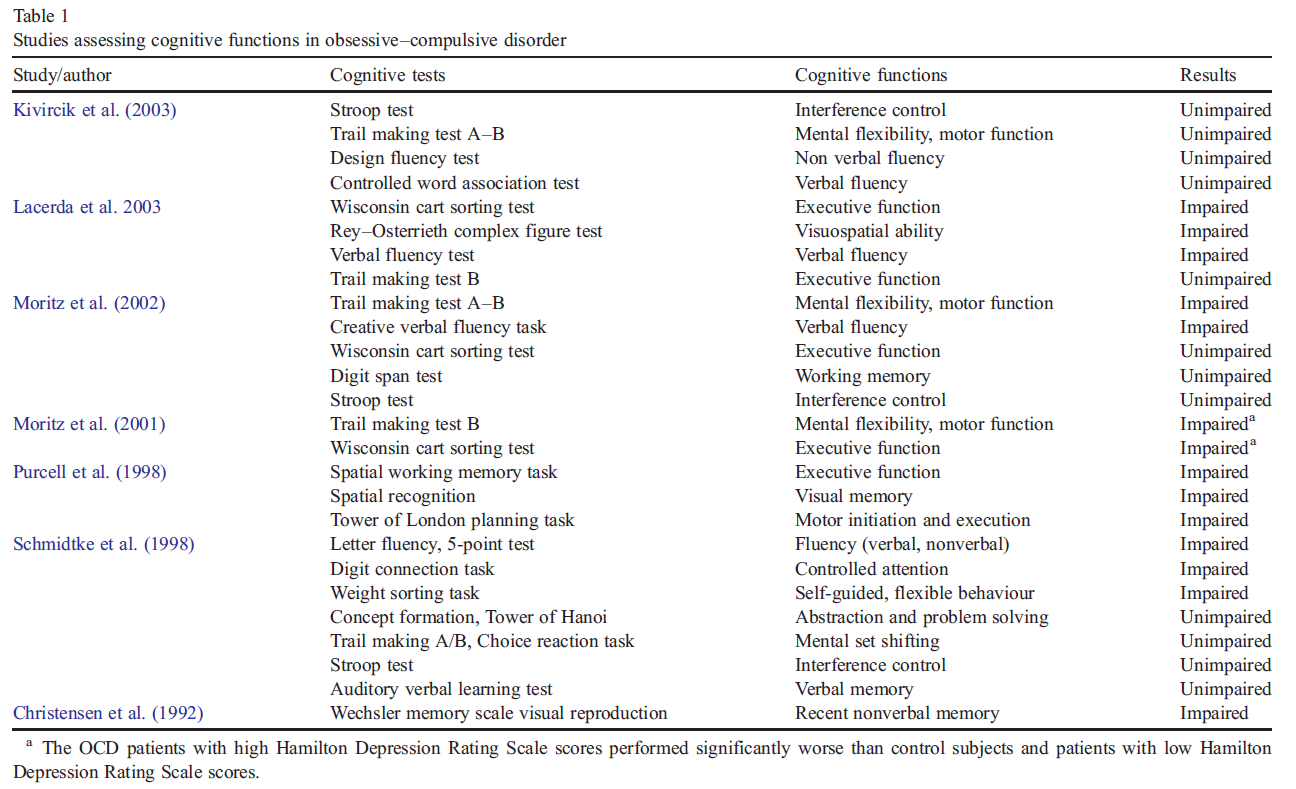
a The OCD patients with high Hamilton Depression Rating Scale scores performed significantly worse than control subjects and patients with low Hamilton Depression Rating Scale scores.
2.Methods
2.1. Subjects
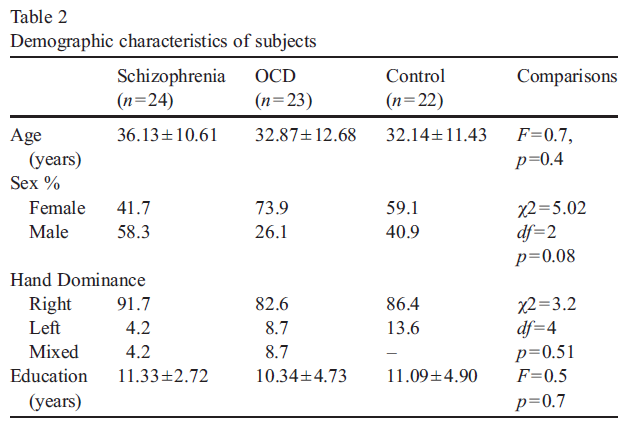
Twenty four patients meeting DSM-IV (American Psychiatric Association, 1994) criteria for schizophrenia and 23 patients meeting those for OCD were included in the study. The control group consisted of 22 healthy subjects matched to the patients in age, gender, education, and in hand dominance. The subjects with apparent or suspected neurological or physical disorders and comorbid psychiatric disorders such as major depression, panic disorder or substance abuse, and those with Hamilton Depression Rating Scale (HDRS) (Williams, 1978) scores above 16 were excluded. The patients had not received any electroconvulsive therapy for the last 6 months. All had at least primary school education. There were no significant differences in gender, age, education, and handedness among three groups (Table 2). There was no significant difference in the duration of illness between OCD group and schizophrenia group.
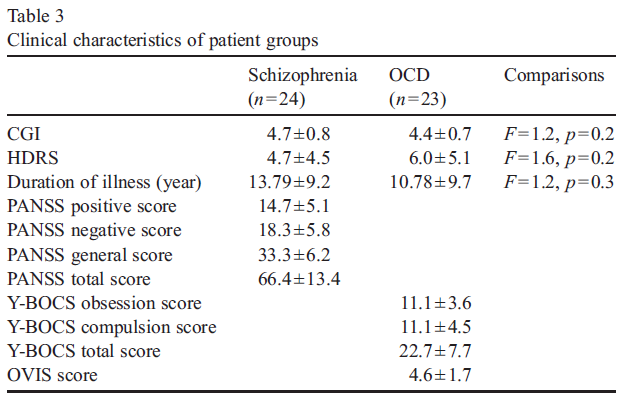
Of the 24 patients with schizophrenia, eight (33.3%) displayed obsessive compulsive symptoms (OCS), predominantly obsessions and compulsions involving a presumed or potential contamination and also compulsions revolving around the issue of control. Two patients in the schizophrenia group had obsessions only: all others had both obsessions and compulsions. Almost all OCD patients experienced both obsessions and compulsions: the most prevalent were those of contamination and those of ag- gressive nature, followed by those involving control issues and counting obsessions. Of the 24 patients with schizophrenia, 2 were drug-free, 12 were on atypical antipsychotics and 8 were on combination of antidepressants and atypical antipsychotics and 2 were on typical antipsychotics. Of the 23 OCD patients, 11 were on antidepressant therapy, 2 were on combination of anti- depressants and antipsychotics and 10 were drug-free. Clinical characteristics of patients are given in Table 3.
All participants gave written informed consent after perusing a full explanation of the study protocol.
2.2. Study instruments
2.2.1 Neuropsychological tests
All of the tests in this battery provide numerous scores, i.e. a multitude of variables. In order to reduce the number of possible dependent variables and to focus the data analysis, a limited subset of the variables were selected a priori.
2.2.1.1. Wisconsin Cart Sorting Test (WCST).
The computer- ized version of WCSTwas administered in our study. The purpose of this test is to assess the patient's ability to form abstract concepts, to utilize feedback, and to shift conceptually (Spreen and Strauss, 1998). The test measures executive functions in that it requires strategic planning, organized searching, the ability to use environmental feedback to shift cognitive sets, goal-oriented behavior, and the ability to modulate impulsive responding. The number of categories completed, and the percent of perseverative errors are the scores in this study.
2.2.1.2. Rey Auditory Verbal Learning Test (RAVLT).
The test consists of five presentations and a recall of a 15 word list, one presentation of a second 15 word list, and a sixth recall trial, with the total duration of 10 to 15 min (Lezak, 1995). A delayed free recall test was performed after a 20-min period, followed by a recognition procedure. The score on each trial is the number of words correctly recalled. The total scores are the sum of words recalled through trials 1 to 5. The number of words recalled after 20 to 30 min provides the delayed recall measure. The recognition discriminability between false and correct words was also asses- sed. The Turkish reliability and validity study of the test was carried out by Acikgoz (1995).
2.2.1.3. Trail-Making Test (TMT).
This test assesses sequencing, attention, mental flexibility, and the speed of visual search and of motor function (Spreen and Strauss, 1998). Part A of this test involves connecting digits from 1 to 25 (dispersed on the on a test sheet) to produce a continuous line that follows the digits in an ascending order. Part B involves alter- nately connecting digits and letters. As Part B was found to be closely related to other tests of timed executive function it is also viewed as a measure of executive function (Libon et al., 1994). The key measures are the time (in seconds) needed by the patient to complete each part of this test.
2.2.1.4. Auditory Consonant Trigram Test (ACTT).
This is a test of short term memory, divided attention, and of information processing capacity and verbal working memory (Spreen and Strauss, 1998). It is also known as the Brown–Peterson Proce- dure. The Turkish reliability and validity study of the test was published by Anil et al. (2003). The score is the sum of all correctly recalled letters.
2.2.1.5. Digit Span Test (DST).
Digit Span Test from the Wechsler batteries is the most commonly used measure of imme- diate verbal recall involving simple items presented in the auditory mode (Lezak, 1995; Wechsler, 1987). The test has two sections: Digits Forward and Digits Backward. In the Forward section the patient repeats the numbers as read to him/her by the examiner (short-term memory is evaluated, via immediate repro- duction). In the Backward section, the patient is instructed to reverse the numbers read to him/her (working memory is eva- luated). The patient's score is the sum of the correct recalled numbers in the forward and backward sections.
2.2.1.6. Controlled Word Association Test (CWAT).
This test evaluates verbal fluency and reasoning. It measures the spontaneous production of words beginning with a given specific letter, as generated within a time limit (Spreen and Strauss, 1998). In English, the F, A, and S are the most commonly used letters for this test. To accommodate the particular characteristics of the Turkish language, the letters K, A, S were used in the Turkish reliability and validity study (Umac, 1997). The scores are the sum of all admissible words for the three letters.
2.2.2. Clinical Assessment Scales:.
Obsessions and compulsions in our OCD group were mea- sured with Yale–Brown Obsessive Compulsive Scale, i.e., Y- BOCS (Goodman et al., 1989a,b; Tek et al., 1995), and Obsession Compulsion Check List, i.e., the OCCL (Goodman et al., 1989a,b). The severity of symptoms of our patients with schizophrenia was assessed with Positive and Negative Syndrome Scale, i.e. the PANSS (Kay et al., 1987; Kostakoglu et al., 1999). Clinical Global Impression Scale (CGI) was used to assess the severity of the disease in both groups of our patients. All patients were also assessed for depressive symptoms via Hamilton Depression Rating Scale (HDRS) (Williams, 1978; Akdemir et al., 2001).
Overvalued ideas were measured with Overvalued Ideas Scale (OVIS). The OVIS (Neziroglu et al., 1999) is an 11-item clinician administered scale to assess the severity of overvalued ideation. Overvalued ideas are assessed on several different continua. These include how strong the obsessive belief is on a scale from 1 (belief is very weak) to 10 (belief is very strong); how reasonable the belief is (1 = totally unreasonable; 10 = completely reason- able); how weak and how strong the belief has fluctuated over the past week (1 = belief is very weak; 10 = belief is very strong); how accurate the belief is (1 = totally inaccurate; 10 = totally accurate); the extent to which others share the same beliefs (1 = totally disagree; 10 = completely agree); how the patient attributes similar (1 = lacking total knowledge, 10: equally as knowledge- able) or differing (1 = equally as knowledgeable 10 = lacking total knowledge) beliefs in other people; how effective and important the compulsions are in preventing negative consequences (1 = totally ineffective and unnecessary; 10 = completely effective and necessary); the extent to which the patient's OCD has caused the obsessive belief (1 = totally probable; 10 = totally improbable); and their degree of resistance toward the belief (1 = total resistance; 10 = no resistance). The average of the first 10 items provides an estimate of one's degree of overvalued ideas, where higher scores represent greater levels of overvalued ideas. The last item identifies the duration of the belief.
Handedness Questionnaire (HQ) was used to assess the lateralization (Chapman and Chapman, 1987; Nalcaci et al., 2002).
3.Statical analysis
2.1. Subjects
The neuropsychological test scores of the OCD, schizophrenia, and control groups were compared via one way analysis of variance (ANOVA). Bonferroni correction was applied in the post-hoc analysis during the multiple comparisons of the three groups. Relationships among the numerous variables were evaluated via Pearson Correlation test. Group differences were also examined defining OCD group based on high OVIS (≥ 5) and low OVIS (b 5) scores. Kruskal–Wallis and MANN Whitney U tests were used for group comparisons where required. The data calculation was carried out with the SPSS program, Version 10.0 for Windows.
4.Results
4.1. Comparison of neuropsychological assessments among three groups
Statistical analysis conducted with one way analysis of variance (ANOVA) revealed a significant difference in WCST, RAVLT, and ACTT scores among the three groups (Table 4). Scores on these three cognitive tests were worse in the schizophrenia group than in the OCD and then in the control group. Schizophrenia group showed significantly lower scores in the category and perseverative error measures on WCST than the OCD group and then the control subjects. Similarly, the schizophrenia group showed significantly worse performance in RAVLT and ACTT, obtaining lower scores for the total of five trials (immediate learning), for the delayed trial and recognition discriminability of RAVLT and also on ACTT total score.
4.1. Comparison of neuropsychological assessments among three groups
There were no significant correlations between neuropsy- chological test scores and HDRS scores in the whole subject group.
The OVIS scores were negatively correlated to WCST category (r =− 0.46, p = 0.03), RAVLT discrimination scores, (r =− 0.43, p = 0.04) and ACTT total scores (r =− 0.44, p = 0.03) in the OCD group. As the OVIS scores increased (indicating an increased prominence of overvalued ideas), the performance deteriorated on WCST, RAVLT and ACTT measures.
In OCD group, there were significant correlations between YBOCS total scores and WCST category (r =− 0.45, p = 0.03), WCST perseverative error (r = 0.51, p = 0.005), RAVLT total score (r =− 0.48, p = 0.02) and Trail-A (r = 0.43, p = 0.04) and B (r = 0.59, p = 0.03) scores.
There were no significant correlations between cognitive test scores and PANSS scores.
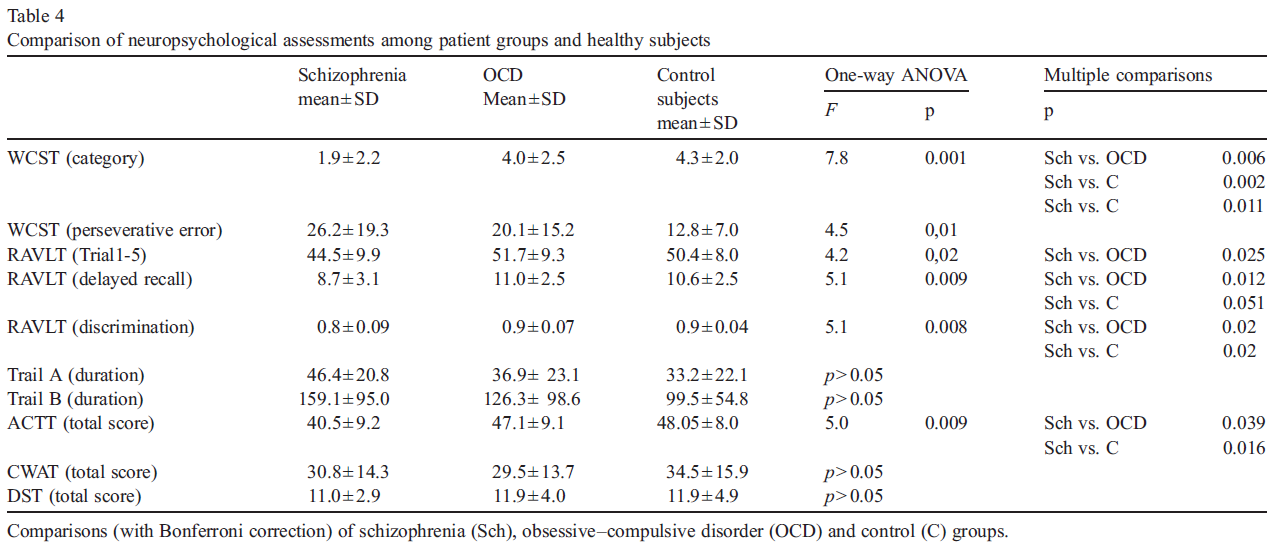
Comparisons (with Bonferroni correction) of schizophrenia (Sch), obsessive–compulsive disorder (OCD) and control (C) groups.
When the schizophrenia patients with obsessive compulsive symptoms (OCS) were compared to those without these symptoms, via Mann Whitney U test, there was no significant difference in cognitive measures between two groups.
4.3. Comparison of cognitive tests among schizophrenia patients, OCD patients with high OVIS scores and low OVIS scores
Our comparison of the patients with schizophrenia and our two groups of OCD patients (those with low and those with high OVIS scores) on cognitive tests, showed no statistically significant difference between the schizophrenia group and OCD patients with high OVIS scores. Our OCD patients with low OVIS scores showed significantly better performance on several cognitive tests, compared to patients with schizophrenia and also compared to OCD patients with high OVIS scores (Table 5). Specifically, OCD patients with low OVIS scores showed better performance on WCST, RAVLT, TMT-A and DST compared to schizophrenia group. The low OVIS group demonstrated better performance on RAVLT than did OCD patients with high OVIS scores.
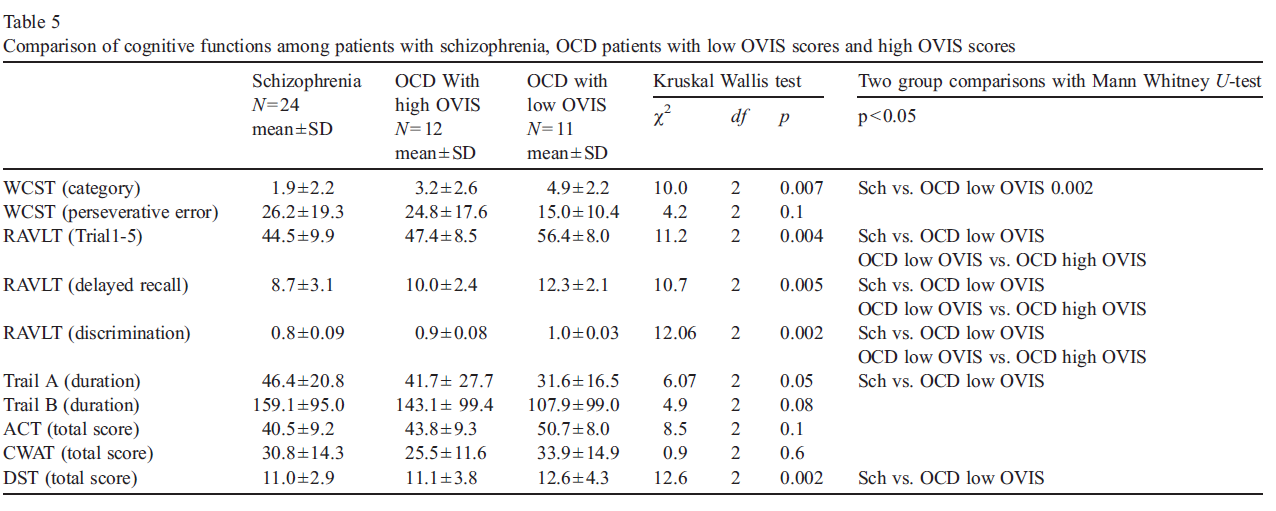
5.Discussion
The patients with schizophrenia showed worse performance on cognitive tests than the OCD group and the control group. Within the OCD group, our correlational analysis showed that the severity of overvalued ideas in OCD may be closely related to cognitive impairments in OCD, especially with respect to executive functions and memory. This particular subtype of OCD (i.e., OCD patients with markedly overvalued ideas) may display similar cognitive dysfunction to schizophrenia.
5.1. Comparison of neuropsychological assessments among three groups
Cognitive dysfunction is one of the key symptoms of schizo- phrenia, in addition to positive and negative symptoms. This study produced results consistent with those found by previous studies of cognitive abnormalities in schizophrenia, i.e., showing deterioration in executive functions, in verbal learning-memory, and in working memory functions. The particular value of our study lies in documenting the difference between this group of patients and the OCD group: the deterioration in cognitive functions was more extensive in the schizophrenia group than in the OCD or in the control group.
Some recent studies found no differences between OCD patients and controls with respect to cognitive functions such as executive functions, attention and memory (Moritz et al., 2002; Kivircik et al., 2003). However, some other studies reported that patients with OCD displayed cognitive dysfunctions (Christensen et al., 1992; Purcell et al., 1998; Schmidtke et al., 1998). This disagreement may be due to the fact that patients with depressive symptoms were included in some studies, or the difference in neurocognitive test batteries used in the various studies, or also to the lack of control for the type of medication used in the treatment of patients in these studies. The present study shares the same limitation for not controlling for medication.
5.2. Relation between cognition and clinical symptoms
In the present study, the cognitive functioning scores of our patients with OCD were not significantly different from those of the control group. However, OVIS scores were strongly correlated to poor cognitive performance in executive functions, verbal learning-memory and working memory. The severity of obsessive–compulsive symptoms was also correlated to performance on executive functions, verbal learning-memory and visuomotor tracking. Group comparisons among schizo- phrenia patients and OCD patients with more prominent overvalued ideas and less prominent overvalued ideas showed significant results. Our OCD patients with more extensive or intensive overvalued ideas (who could perhaps be labeled as “schizo-obsessive subtype of OCD”) displayed more extensive cognitive dysfunctions on tests of verbal learning and memory than OCD patients with lower scores for overvalued ideas. In addition, the OCD patients with more severe overvalued ideas were not different from schizophrenia patients in means of cognition while those with less severe overvalued ideas were significantly better on cognitive tests of executive functions, verbal-learning-memory, attention and visuomotor tracking compared to schizophrenia patients.
The cognitive functions in the schizophrenia group were not correlated to the severity of the disease and the schizophrenia patients with obsessive compulsive symptoms (OCS) did not differ from those without, in means of cognition. These results were contradictory to the results of the studies showing relation between OCS and cognition in patients with schizophrenia (Hwang et al., 2000; Lysaker et al., 2000, 2002). In our sample of schizophrenia patients, OCS appeared not to have influence on cognition.
None of the previous studies has measured the effect of overvalued ideas on cognitive functions in OCD. In fact, the differences among the various samples in these studies with respect to the relative proportions of patients with overvalued ideas within each of these samples of OCD patients may influence, as a confounding factor, the differences on neuro- cognitive test scores. The present study found that overvalued ideas in the OCD group were positively correlated to executive functions and working memory.
Since OCD patients with co-morbid depression were not included in our study, the number of the patients with OCD was limited. Hence the usual negative effect of depression on cognitive function was prevented. Depression was reported to have negative effects on cognitive functions in patients with OCD (Cox, 1997; Moritz et al., 2002). It should be noted that while in the study by Moritz et al. (2001) OCD patients with more depressive symptoms displayed more cognitive dysfunction than control subjects, OCD patients with lower levels of depressive symptoms did not significantly differ from the controls in their cognitive functioning scores.
Westpal (1978) suggested that obsessive–compulsive symptoms might be one of the signs of schizophrenia or a part of its whole spectrum, and defined OCD as an abortive insanity by pointing at the similarities between the OCD and psychosis in terms of thought disorder. Rosen (1957) proposed that, in some of the schizophrenia patients showing obsessive symptoms, these symptoms may either precede or coincide with schizophrenia and he also described a transition from obsession to delusion in some of these patients. OCD patients with overvalued ideas may differ from OCD patients without overvalued ideas with respect to the extent of their cognitive dysfunction. A person with OCD typically regards obsessions as illogical and resists compulsions. However, there are also those whose insights deteriorate and those who do not resist their compulsions. These schizophreniform symptoms are called schizo-obsessive (Hwang and Hollander, 1993) or obsessive psychosis (Solyom et al., 1985). Therefore schizophrenia and OCD may be viewed as two extreme ends of the same spectrum.
Foa (1979), viewed obsessions as intellectually insignificant and proposed that resisting obsession causes more problems than their acceptance. Yaryura-Tobias places the overvalued idea in the continuum between obsessions and delusions and emphasizes that this is an intensive belief, stronger than an obsession but weaker than a delusion (Yaryura-Tobias and Neziroglu, 1997).
The use, in DSM-IV, of the additional specifier “with poor insight” for certain forms of OCD increases the theoretical complexity (see DSM-IV, 1994). In fact, this term is only used when a person considers his obsessions or compulsions neither as extreme nor as illogical during most of the current episode. Obsession and delusion are at two ends of the same spectrum as a cognitive dysfunction and may transform from one to the other (Insel and Akiskal, 1986). An overvalued idea, stronger than an obsession but weaker than a delusion, is a belief in which a patient assumes that his fear and obsession are realistic, and this leads to the patient's decision not to resist but to accept the obsession. When regarded as a transformation or evolution of an obsessive idea, the overvalued ideas can be seen as an interim step of transition from obsession to delusion. Therefore the term impaired “insight” in DSM-IV could perhaps be, in some manner, replaced with the concept of “overvalued idea”. The OCD group with overvalued ideas is closer to schizophrenia,and, therefore, cognitive dysfunction in this group is more salient. Overvalued ideas have a predictive prognostic value in measuring outcomes of OCD (Neziroglu et al., 2001). This study shows that schizophrenia may differ from OCD with respect to the extent of cognitive dysfunction. Our results also show that the OCD patients with overvalued ideas are closer to schizophrenia patients in terms of cognitive functioning than are OCD patients without overvalued ideas.
6.Conclusion
OCD with overvalued ideas may resemble OCD with psychotic features and may be related to “schizo-obsessive subtype” of OCD. The present study investigated the role of overvalued ideas with respect to neurocognitive functioning. OCD patients with overvalued ideas showed similar cognitive functions as the patients with schizophrenia.
References
- Abbruzzese M, Bellodi L. Frontal lobe dysfunction in schizophrenia and obsessive–compulsive disorder: a neuropsychological study. Brain Cogn 1995; 27:202–12.
- Abbruzzese M, Ferri S, Scarone S. The selective breakdown of frontal functions in patients with obsessive–compulsive disorder and in patients with schizophrenia: a double dissociation experimental finding. Neuropsycholo- gia 1997; 35:907–12.
- Acikgoz DG. Evaluation of factorial structure of the neuropsychological tests measuring memory and attention in statistical and descriptive means. Institute of Social Sciences at Hacettepe University, 1995; Master Dissertation.
- Akdemir A, Turkcapar MH, Orsel SD, Demirergi N, Dag I, Ozbay MH. Reliability and validity of the Turkish version of the Hamilton Depression Rating Scale. Compr Psychiatry 2001; 42:161–5.
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. Fourth Edition. DC: Washington; 1994.
- Andreasen NC, O'leary DS, Cizaldo T, Arndt S, Rezai K, Ponto LLB, et al. Schizophrenia and cognitive dysmetria: a positron-emission tomography study of dysfunctional prefrontal–thalamic–cerebellar circuitry. Proc Natl Acad Sci 1996; 93:9985–90.
- Andreasen NC, Nopoulos P, O'Leary DS, Miller DD, Wassink T, Flaum M. Defining the phenotype of schizophrenia: cognitive dysmetria and its neural mechanisms. Biol Psychiatry 1999; 46:908–20.
- Anil AE, Kivircik B, Batur S, Kabakci E, Kitis A, Guven E, et al. The Turkish version of the auditory consonant trigram test as a measure of working memory: a normative study. Clin Neuropsychol 2003; 17:159–69 [May].
- Bilder RM, Goldman RS, Robinson D, Reiter G, Bell L, Bates JA, et al. Neuropsychology of first episode schizophrenia: initial characterization and clinical correlates. Am J Psychiatry 2000; 157:549–59.
- Bland RC, Newman SC, Orn H. Schizophrenia: lifetime comorbidity in a community sample. Acta Psychiatr Scand 1987; 75:383–91.
- Chapman LJ, Chapman JP. The measurement of handedness. Brain Cogn 1987; 6:175–83.
- Christensen KJ, Kim SW, Dyksen MW, Hoover KM. Neuropsychological performance in obsessive–compulsive disorder. Biol Psychiatry 1992; 31:4-18.
- Cox CS. Neuropsychological abnormalities in obsessive–compulsive disorder and their assessments. Int Rev Psychiatry 1997; 9:45–59.
- Eisen JL, Beer DA. Obsessive–compulsive disorder in patients with schizophrenia or schizoaffective disorder. Am J Psychiatry 1997; 154:271–3.
- Eisen JL, Rasmussen SA. Obsessive–compulsive disorder with psychotic features.J Clin Psychiatry 1993;54(10):373–9.
- Fenton WS, McGlashan TH. The prognostic significance of obsessive– compulsive symptoms in schizophrenia. Am J Psychiatry 1986; 143:437–41.
- Foa EB. Failure in treating obsessive–compulsives. Behav Res Ther 1979;17:169–76.
- Gold J, Carpenter C. Auditory working memory and Wisconsin Cart Sorting Test performances in schizophrenia. Arch Gen Psychiatry 1997; 54:159–65.
- Goodman WK, Price LH, Rasmussen SA, Mazure C, Delgado P, Heninger GR, et al. The Yale–Brown Obsessive–Compulsive Scale, II: validity. Arch Gen Psychiatry 1989a; 46:1012–6.
- Goodman WK, Price LH, Rasmussen SA, Mazure C, Fleischmann RL, Hill CL, et al. The Yale–Brown Obsessive–Compulsive Scale I: development, use and reliability. Arch Gen Psychiatry 1989b;46:1006–11. Hwang MY, Hollander E. Schizo-obsessive disorders. Psychiatr Ann 1993;23 (7):396–401.
- Hwang MY, Morgan JE, Losconzcy MF. Clinical and neuropsychological profiles of obsessive–compulsive schizophrenia: a pilot study. J Neuropsy- chiatry Clin Neurosci 2000;12(1):91–4.
- Insel TR, Akiskal HS. Obsessive–compulsive disorder with psychotic features: a phenomenologic analysis. Am J Psychiatry 1986; 143:1527–33.
- Kay SR, Fiszbein A, Opler LA. The Positive and Negative Syndrome Scale (PANSS) for schizophrenia. Schizophr Bull 1987; 13:261–76.
- Kivircik BB, Yener GG, Alptekin K, Aydın H. Event-related potentials and neuropsychological tests in obsessive–compulsive disorder. Prog Neurop- sychopharmacol Biol Psychiatry 2003; 27:601–6.
- Kostakoglu AE, Batur S, Tiryaki A. The validity and reliability of the Turkish version of the Positive and Negative Syndrome Scale (PANSS). Turk J Psychol 1999; 14:23–32.
- Kozak MJ, Foa EB. Obsessions, overvalued ideas, and delusions in obsessive- compulsive disorder. Behav Res Ther 1994; 32:343–53.
- Lacerda ALT, Dalgalarrondo P, Caetano D, Haas GL, Camargo EE, Keshavan MS. Neuropsychological performance and regional cerebral blood flow in obsessive–compulsive disorder. Prog Neuropsychopharmacol Biol Psychiatry 2003; 27:657–65.
- Lelliott PT, Noshirvani HF, Basoglu M, Marks IM, Monteiro WO. Obsessive– compulsive beliefs and treatment outcome. Psychol Med 1988; 18:697–702.
- Lezak MD. Neuropsychological assessment. Third edition. New York: Oxford University Press; 1995.
- Libon DJ, Glosser G, Malamut BL, Kaplan E, Goldberg E, Swenson R, et al. Age, executive functions, and visuospatial functioning in healthy older adults. Neuropsychology 1994; 8:38–43.
- Lysaker PH, Marks KA, Picone JB, Rollins AL. Obsessive and compulsive symptoms in schizophrenia: clinical and neurocognitive correlates. J Nerv Ment Dis 2000; 188:78–83.
- Lysaker PH, Bryson GJ, Marks KA, Greig TC, Bell MC. Association of obsessions and compulsions in schizophrenia with neurocognition and negative symptoms. J Neuropsychiatry Clin Neurosci 2002;14(4):449–53.
- Moritz S, Birkner C, Kloss M, Fricke S, Böthern A, Hand I. Impact of comorbid depressive symptoms on neuropsychological performance in obsessive– compulsive disorder. J Abnorm Psychol 2001; 110:653–7.
- Moritz S, Birkner C, Kloss M, Jahn H, Hand I, Haasen C, et al. Executive functioning in obsessive–compulsive disorder, unipolar depression, and schizophrenia. Arch Clin Neuropsychol 2002; 17:477–83.
- Nalcaci E, Kalaycioglu C, Gunes E, Cicek M. Reliability and validity of a Handedness Questionnaire. Turk J Psychiatry 2002; 13:99-106.
- Neziroglu F, Yaryura-Tobias JA. Over and over again: understanding obsessive compulsive disorder (updated and revised). New York: Lexington Books; 1995.
- Neziroglu F, McKay D, Yaryura-Tobias JA, Stevens KP, Torado J. The overvalued ideas scale: development, reliability and validity in obsessive compulsive disorder. Behav Res Ther. 1999; 37:881–902.
- Neziroglu F, Stevens KP, McKay D, Yaryura-Tobias JA. Predictive validity of the overvalued ideas scale: outcome in obsessive–compulsive and bodydysmorphic disorders. Behav Res Ther 2001; 39:745–56.
- Payurovsky M, Fuchs Camil. Obsessive–compulsive disorder in patients with first-episode schizophrenia. Am J Psychiatry 1999; 156:1998–2000.
- Payurovsky M, Kriss V, Weisman G, Faragian S, Kurs R, Schneidman M, et al. Comparison of clinical characteristics and comorbidity in schizophrenia patients with and without obsessive compulsive disorder: schizophrenic and OC symptoms in schizophrenia. J Clin Psychiatry 2003; 64:1300–7.
- Purcell R, Maruff P, Kyrios M, Pantelis C. Neuropsychological defects in obsessive–compulsive disorder: a comparison with unipolar depression, panic disorder, and normal controls. Arch Gen Psychiatry 1998; 55:415–23.
- Reitan RM. Trail making test. Manual for administration and scoring. South Tuscon: AZ. Reitan Neuropsychology Laboratory; 1993.
- Rosen I. The clinical significance of obsessions in schizophrenia. J Ment Sci 1957; 103:773–85.
- Saykin AJ, Shtasel DL. Neuropsychological deficits in neuroleptic naive patients with first-episode schizophrenia. Arch Gen Psychiatry 1994; 51:124–31.
- Schmidtke K, Schorb A, Winkelmann G, Hohagen F. Cognitive frontal lobe dysfunction in obsessive–compulsive disorder. Biol Psychiatry 1998;43 (9):666–73.
- Solyom L, Dinicola VF, Phil M, Sookman D, Luchins D. Is there an obsessive psychosis? A etiological and prognostic factors of an atypical form of obsessive–compulsive neurosis. Can J Psychiatry 1985; 30:372–9.
- Spreen O, Strauss E. A compendium of neuropsychological tests: administration, norms, and commentary. Second edition. New York: Oxford University Press; 1998.
- Tek C, Ulug B, Rezaki BG, Tanrıverdi N, Mercan S, Demir B, et al. Yale–Brown Obsessive–Compulsive Scale and US National Institute of Mental Health Global Obsessive Compulsive Scale in Turkish: reliability and validity. Acta Psychiatr Scand 1995; 91:410–3.
- Tibbo P, Warneke L. Obsessive–compulsive disorder in schizophrenia: epidemiologic and biologic overlap. J Psychiatry Neurosci 1999; 24:15–24.
- Umac A. The influence of age and education to the tests severe sensitive to frontal lesions in normal subjects. Institute of Social Sciences at Istanbul University, Master Dissertation; 1997.
- Wechsler D. Wechsler Memory Scale—revised. San Antonio, TX: The Psycholog- ical Corporation; 1987.
- Westpal K. Ueber Zwangsvorstellungen. Arch Psychiatr Nervenkr 1978; 8:734–50.
- Williamss BW. A structured interview guide for Hamilton Depression Rating Scale. Arch Gen Psychiatry 1978; 45:742–7.
- Yaryura-Tobias JA, Neziroglu FA. Obsessive–compulsive disorder spectrum: pathogenesis, diagnosis, and treatment. Washington, USA: American Psychi- atric Press, Inc.; 1997. Chapter 5.